In the dental implant restoration supply chain, over 70% of remake cases stem from technical deficiencies or improper data handling, issues that not only increase costs but also extend average delivery times by 3–5 days. To ensure consistent restoration quality, reliable turnaround, and a stable long-term partnership, procurement teams should evaluate potential lab partners against measurable technical and operational criteria:
- Digital workflow readiness – CAD/CAM proficiency, STL file compatibility, and secure cloud-based submission and tracking systems.
- Advanced production equipment – High-precision 3D printing, modern milling/scanning systems, and disciplined maintenance schedules.
- Material expertise & compliance – ADA-compliant ceramics and composites, verified ISO/CE/FDA certifications, and third-party quality test reports.
- Quality control strength – Multi-stage inspection processes, KPI tracking for remake rates, and sample case validation before bulk production.
- Collaboration & support – Clear case specification templates, a dedicated QC liaison, and structured real-time feedback channels.
- System adaptability & customization – Experience across multiple implant systems and tailored design solutions for complex cases.
- Partnership reliability – Transparent pricing models, realistic turnaround commitments, ongoing training investment, and verifiable client references.
Focusing on these capabilities will help secure predictable quality, minimize costly remakes, and build a dependable overseas dental lab partnership that supports sustainable growth in the implant restoration supply chain.
How to Evaluate a Lab’s Digital Workflow Capabilities
A lab’s digital workflow determines how efficiently and accurately it can translate clinical data into finished implant restorations. Evaluating CAD/CAM proficiency, system compatibility, and data-handling protocols helps procurement teams select partners who can deliver consistent quality while minimizing errors.

dental-lab-digital-workflow-evaluation
Assessing CAD/CAM proficiency and STL file compatibility
- Evaluate design accuracy – Request sample cases designed by the lab to assess margin definition and occlusal mapping.
- Confirm file compatibility – Ensure the lab’s CAD/CAM systems work seamlessly with your scanners and preferred file formats (STL, PLY, or proprietary).
- Test transfer workflows – Send test files to see if the lab can open, process, and return designs without conversion errors.
Integration of digital scanning and design into the production process
A North American distributor we support reduced communication delays by partnering with a lab that directly integrated intraoral scanning into its CAD/CAM workflow. Cases moved from scan to design within hours, cutting the average turnaround time by two days. This integration also minimized manual data entry, reducing the risk of mismatched case details.
Using cloud-based submission and tracking tools for error prevention
- Select a secure, compliant platform – Choose HIPAA or GDPR-compliant systems to protect patient data.
- Enable automated acknowledgements – Both parties should receive instant confirmation when files are uploaded.
- Use shared progress tracking – Allow real-time status updates so clinicians and procurement teams can monitor case stages.
Digital workflow capability is a strong predictor of a lab’s ability to deliver accurate, timely results. Partnering with a lab that offers robust CAD/CAM integration, proven file compatibility, and reliable cloud-based tools can significantly reduce remakes and improve collaboration.
What Advanced Technologies and Equipment Indicate Strong Production Capability
The technologies and equipment a lab uses directly influence the precision, speed, and consistency of implant restorations. Evaluating a partner’s hardware infrastructure provides insight into their ability to handle complex cases, maintain high quality, and meet demanding timelines.

dental-lab-advanced-equipment-3dprinting-milling
Role of 3D printing in model creation and restoration fabrication
3D printing has transformed model production, enabling same-day fabrication of highly accurate models for case planning and verification. Labs equipped with dental-grade printers can also produce resin patterns for casting or temporary restorations, reducing reliance on outsourcing and accelerating turnaround. The ability to print in multiple materials—such as biocompatible resins and high-resolution model resin—adds flexibility in handling a variety of implant cases.
Modern milling, scanning, and finishing systems for precision work
- High-precision milling units – Capable of producing zirconia, titanium, and PMMA restorations with micron-level accuracy.
- Advanced scanning systems – Ensure detailed capture of implant geometries, enabling precise fit and occlusion.
- Automated finishing tools – Deliver consistent polishing, glazing, and texturing for a natural aesthetic.
How equipment maintenance affects consistency and turnaround
A South American distributor we partner with noticed a drop in remake rates after their chosen lab implemented a preventive maintenance program. Regular calibration of milling units, scheduled replacement of scanner components, and adherence to manufacturer service intervals ensured consistent production output. This not only improved quality but also minimized unexpected downtime that could delay critical cases.
Choosing a lab with advanced, well-maintained equipment is an investment in reliability. According to dental manufacturing technology standards, facilities that integrate modern production tools and maintain them rigorously are better positioned to deliver precise, timely restorations at scale.
How to Verify a Lab’s Material Expertise and Standards Compliance
A lab’s material expertise directly impacts the safety, durability, and aesthetics of implant restorations. From ceramic selection to compliance with global health standards, verifying these capabilities is essential for ensuring predictable, high-quality outcomes.

dental-lab-materials-standards-certificates
Knowledge of ceramics, composites, and implant components
- Ceramics – Zirconia and e.max lithium disilicate for strength and translucency.
- Composites – High-strength hybrid composites for provisional or cost-sensitive cases.
- Implant components – Titanium and titanium alloy abutments engineered for biocompatibility and precision fit.
Verifying ADA, ISO, CE, and FDA compliance for materials used
- Request official certificates – Ensure documents are issued by accredited certification bodies.
- Check scope of approval – Verify that listed materials are approved for dental and implant applications.
- Confirm audit and renewal dates – Outdated or lapsed certificates may signal process lapses.
Requesting third-party validation reports for material quality
One European procurement team we work with requires independent lab testing for every new material batch—covering flexural strength, wear resistance, and biocompatibility. This practice not only safeguards patient outcomes but also provides leverage in supplier negotiations, as it sets a measurable benchmark for acceptance.
By confirming a lab’s expertise and compliance, procurement teams can ensure their restorations meet both performance expectations and regulatory requirements. Partnering with a certified dental materials supplier helps maintain consistency and builds trust in long-term collaboration.
What Quality Control Processes Reduce Errors and Remakes
An effective quality control (QC) process ensures every implant restoration meets fit, function, and aesthetic requirements before it leaves the lab. By combining multi-stage checks, KPI tracking, and data-driven improvement measures, procurement teams can reduce remakes and strengthen supplier reliability.
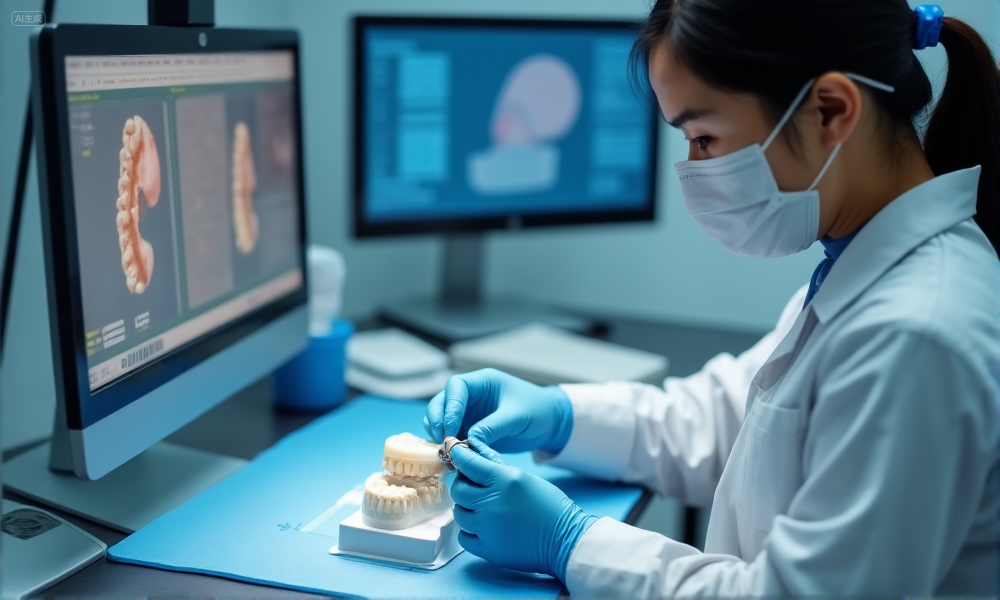
dental-lab-quality-control-process
Multi-stage QC checks from design to final restoration
- Digital design review – Confirm margins, occlusion, and implant interface before production.
- Mid-production inspection – Verify milling accuracy and surface integrity after initial fabrication.
- Final restoration check – Inspect shade, surface finish, and fit before packaging and shipment.
KPI tracking for remake rate and accuracy performance
- Remake percentage – Total remakes vs. total cases handled.
- First-time fit rate – Percentage of restorations requiring no adjustment upon delivery.
- On-time delivery rate – Cases shipped within the agreed timeline.
Reviewing remake data and case outcomes for continuous improvement
One Asia-Pacific distributor reduced remakes by 25% after partnering with us to conduct quarterly case reviews. By analyzing remake data, we identified a recurring fit issue linked to specific scan sources, enabling targeted adjustments in both clinic scanning protocols and lab processing steps.
Trial runs and sample case validation before bulk production
Running small-scale trial cases before full-scale production helps detect compatibility issues, workflow bottlenecks, and material inconsistencies without jeopardizing major orders. This approach builds a solid foundation for consistent results in bulk production.
Strong QC processes not only reduce costly errors but also build long-term trust. Partnering with a lab that prioritizes measurable quality metrics, data analysis, and sample-based validation—as outlined in dental laboratory QA guidelines—provides procurement teams with predictable outcomes and fewer operational disruptions.
Why Communication and Technical Support Are Critical for Collaboration
Clear, responsive communication and strong technical support ensure that implant restoration projects stay on track, meet specifications, and resolve issues quickly. These capabilities strengthen the working relationship between procurement teams and lab partners, reducing misunderstandings and costly delays.
dental-lab-client-collaboration-video-meeting
Setting clear case specifications and feedback protocols
- Define clinical and technical requirements upfront – Include margin design, material selection, and shade references.
- Use a standardized case submission form – Reduce ambiguity by capturing all necessary details in one document.
- Agree on feedback timelines – Ensure revisions or clarifications are addressed promptly to avoid production delays.
Assigning a dedicated QC or technical liaison for each account
One European procurement group improved turnaround predictability by working with a lab that assigned a dedicated QC manager. This point of contact handled all case-related queries, tracked progress, and provided proactive updates—minimizing back-and-forth communication and improving issue resolution speed.
Establishing structured digital feedback loops for ongoing cases
- Cloud-based comments on CAD designs – Allows real-time feedback before milling or printing.
- Shared case status dashboards – Keeps all stakeholders updated on progress.
- Post-delivery review forms – Captures issues for process improvement.
Providing rapid technical troubleshooting for complex cases
Complex implant cases often require immediate input from an experienced technician. Labs offering same-day technical consultations—via video call or annotated design screenshots—help clinicians make informed decisions quickly, avoiding costly chairside adjustments.
Strong communication channels and dependable technical support build a foundation of trust and efficiency. Partnering with an overseas dental lab that offers structured feedback systems and responsive troubleshooting ensures smoother workflows and better long-term results.
How System Experience and Customization Capabilities Add Value
A lab’s ability to work across multiple implant systems and provide tailored design solutions gives procurement teams greater flexibility in meeting diverse clinical needs. This adaptability not only broadens case compatibility but also ensures consistent quality regardless of system or complexity.
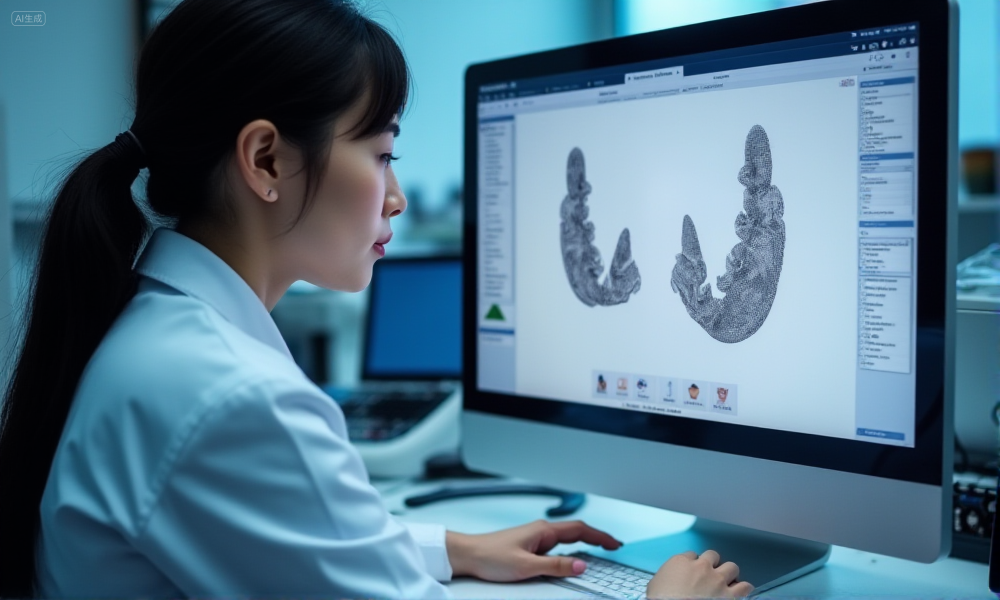
dental-lab-multi-system-customization
Adapting workflows to multiple implant systems and restorative needs
Experienced labs can seamlessly adjust their workflows for different implant platforms—whether it’s Nobel Biocare, Straumann, or custom abutments. This includes modifying scan body libraries, adjusting milling parameters, and tailoring finishing techniques to match each system’s unique specifications.
Offering customized design solutions for special case requirements
- Anatomically driven designs – Customized contours for complex occlusal schemes.
- Hybrid prosthetics – Combining materials like zirconia frameworks with composite layering for aesthetic and functional balance.
- Case-specific emergence profiles – Adjusting soft tissue support for optimal healing and long-term stability.
Balancing flexibility with consistent quality output
One North American procurement partner we support handles cases from multiple clinics, each preferring different implant systems. By working with a lab experienced in cross-platform workflows, they achieved consistent turnaround times and maintained a uniform standard of quality—regardless of system complexity—without adding internal coordination overhead.
Versatility in system handling and customization translates to better clinical outcomes and smoother procurement operations. Partnering with a global dental lab skilled in multi-system workflows ensures every case receives the same level of precision and attention to detail.
Additional Factors to Ensure a Reliable Long-Term Partnership
Beyond technical capabilities and quality processes, sustaining a dependable partnership with your implant lab requires clear agreements, mutual trust, and shared commitment to improvement. These factors ensure smooth operations over years of collaboration.

dental-lab-long-term-partnership
Balancing cost-effectiveness with quality assurance measures
Long-term partnerships thrive when both parties agree on pricing models that deliver value without sacrificing quality. This balance often includes volume-based discounts while maintaining rigorous QC protocols to protect clinical outcomes.
Setting clear and realistic turnaround time commitments
- Define service level agreements (SLAs) – Establish delivery timelines for different restoration types.
- Build flexibility for urgent cases – Include provisions for rush orders without impacting regular workflows.
- Monitor on-time delivery performance – Track and review turnaround compliance quarterly.
Maintaining continuous training and adopting new technologies
Labs that invest in ongoing technician training and equipment upgrades are better equipped to handle evolving implant systems, new materials, and emerging clinical techniques. This ensures their capabilities remain aligned with the latest market demands.
Requesting peer testimonials or case references for credibility
Hearing from other procurement teams or clinics who have maintained successful partnerships with the lab provides reassurance of their reliability. These testimonials and case references can also highlight the lab’s strengths in communication, quality, and delivery consistency.
By aligning on cost, timelines, innovation, and credibility, procurement teams can secure partnerships that endure. Collaborating with an overseas dental lab that values both performance and long-term relationship-building creates a foundation for consistent quality and mutual growth.
Conclusion
Building a successful implant restoration supply chain requires more than technical capability—it demands trust, clear communication, and consistent quality. By selecting a partner with proven digital workflows, advanced equipment, strong material compliance, and measurable QC processes, procurement teams can reduce remakes, improve efficiency, and maintain predictable outcomes. Collaborating with an overseas dental lab that adapts to multiple systems, invests in innovation, and prioritizes long-term relationships ensures every case meets both clinical and operational goals, strengthening the foundation for sustained growth and mutual success.


